This article is co-written by biologist Iida Ruishalme (yours truly at Thoughtscapism) and neuroscientist Alison Bernstein, aka Mommy PhD from SciMoms.

In this piece we dive into the wealth of information available on toxicity, and take a closer look at two main categories: acute and chronic toxicity. Large versions of the infographics separately below.
We live amidst a mind-bogglingly rich sea of molecules. Nowadays, we also have astonishingly sophisticated methods of chemical detection at our disposal, and are able to measure smaller and smaller traces of substances in our environment. This is great! We can learn to understand molecular interactions better than ever before, and with the help of this information we can also better monitor and regulate potentially harmful exposures.
But when we know, we worry. Sometimes this wealth of knowledge leads to undue fear of substances even when they are present in minute quantities that pose little risk and a wish to remove these traces altogether. However, trying to remove all traces of unwanted substances in our environment is an impossible goal. As we have written before:
We often strive for choices with zero risk. However, zero risk is an impossible goal. Certain activists and consumers seem to want an even more conservative goal of zero exposure, whether there is risk or not. Zero risk and zero exposure are impossible goals. Nearly everything we do has both risks and benefits. Everything, even inaction, carries risk. Thus, decisions, both personal and regulatory, are a matter of balancing the relative risks and benefits of your choices and choosing the level of risk you find acceptable, rather than of trying (and inevitably failing) to avoid all risk and all exposure to hazards.
An absolutely ‘clean’ state of being is a fantasy. Life itself is a ‘messy’ chemical phenomenon, which has arisen in and continues to adapt to environments that vary greatly in chemical composition. Biology is an act of balancing a mix of millions of molecules in proportions that enable the continued functioning of body processes like homeostasis, protein synthesis, and self-replication. When a dose of a substance is high enough to disturb these dynamics in a living organism in some significant way, it is considered toxic.
In order to measure risk, scientists must first establish metrics to define a level that represents minimal risk. These metrics generally fall into two groups: acute toxicity metrics and chronic toxicity metrics.
Acute toxicity
Acute toxicity is the kind of harm which describes classical poisoning effects. People often compare measures of acute toxicity expressed as LD50, which measures lethal effects from a large one-time dose, when trying to place these exposures in context. As the famous quote goes, “the dose makes the poison” (see Dr Cami Ryan’s version of an acute toxicity comparison with that very title here). However, as noted by Alison in a previous piece:
Let’s get something straight about LD50 – it is a measure of ACUTE toxicity. That is, LD50 is relevant for accidents, murders or suicides.
An LD50, or the median Lethal Dose, and the related LC50 (median lethal concentration, for inhalation rather than ingestion) are measures of acute toxicity only. Acute toxicity relates to adverse effects that occur after a single exposure or multiple exposures within a day, and effects that manifest immediately or within two weeks of the exposure. The LD50 is determined experimentally, usually with rats or mice. It is single acute dose that will kill 50% of a population given that dose. If you have a test population of 100 rats, it is the dose found to be sufficient to kill 50 of them. Likewise, the LD50 for humans is the dosage of a compound estimated that would kill 50 out of 100.
LD50s tell us about risk in cases where someone is exposed to a large amount of a chemical in a short amount of time. In other words: accidents, murders or suicides.

Source links for the values can be found in the end of the piece.
One way we can learn more about chemicals we don’t know, is to put them into context by comparing them with more familiar exposures. Acute toxicity comparisons are helpful for reminding us that any substance can cause great harm if the dose is high enough. However, as Alison writes:
Most real human exposures are not acutely lethal but have other, long-term or chronic, effects that may or may not be toxic. Thus, LD50s are not very useful when considering health effects of the large majority of human exposures.
And that isn’t even the only drawback with the LD50 measurement:
The use of LD50s is outdated in toxicology
The classic LD50 experiment and the use of the metric itself have several drawbacks:
- the fatality rates may vary from experiment to experiment due to diet, genetics, and many other factors
- lethal doses may differ between species (what is poisonous to dogs may not be so poisonous to us, say);
- there are major ethical problems with killing a large number of lab animals for ambiguous or nonessential information.
Essentially, the data from these experiments are unreliable and not very useful, making the ethics of these experiments highly problematic.
You can read more about the criticism of LD50s in a 1981 paper, Significance of the LD50-test for the toxicological evaluation of chemical substances. Most developed countries abolished the requirement for LD50 testing in 2001 by OECD agreement, replacing it with tests that use far fewer animals with lower, non-lethal doses. Alternate methods of acute toxicity testing include the Up Down Procedure, Acute Toxic Class Method, and others, described in greater detail in this post at Compound Interest. Some acute toxicity values are now reported as >5000 mg/kg, a dose level at which tests will no longer proceed.
Unless the intended use of a chemical is to instantly kill (say, rat poison), exactly how much of the substance is lethal to a certain portion of various mammals is not a very useful measurement. Is it really so important to know what dose of a substance will have an 50-50 chance of killing an animal if we already know it will cause health problems at a much lower dose?
Chronic toxicity
Outside of cases of acute poisoning, most of the time we are interested in finding the lowest level of daily exposure that causes harm. As mentioned above, LD50 values give us very little information about these long-term effects. Instead, chronic toxicity metrics are based on the “Lowest Observable Adverse Effects Level” (LOAEL) and the “No Observable Adverse Effects Level” (NOAEL). These are experimentally determined metrics defined as the lowest dose at which adverse effects are seen (LOAEL) or the dose at which no adverse effects are seen (NOAEL). These measures are much more useful in guiding regulations and personal choices to ensure that we avoid adverse health effects – whether it be an an increased risk of cancer, heart disease, neurodevelopmental problems, or other adverse effects.
The daily limits set through assessments by regulatory agencies are based on these NOAELs or LOAELs. These metrics are estimates of the daily exposure to humans that is likely to be without appreciable risk of deleterious effects throughout the entire lifetime. These are typically derived by dividing the NOAELs or LOAELs by a set of uncertainty factors (for more details see Alison’s piece how these are calculated). Examples of these chronic toxicity metrics include:
- Reference Dose (RfD, in the US) especially for pesticides
- Acceptable Daily Intake (ADI, in the EU) for food additives, pesticides, and drugs
- Tolerable Daily or Weekly Intakes (TDI or TWI) for contaminants not used intentionally
- Tolerable upper intake levels (UL) in connection to Dietary Reference Intake (DRI) information for foodstuffs, minerals, and vitamins
- Reference Intakes (RI) for daily nutrient recommendations in the EU
For a detailed discussion on how the RfD and ADI limits are determined, you can read Alison’s piece Glyphosate and Caffeine: Acute and Chronic Toxicity Assessments Explained.

Sources for the values can be found in the end of the piece. Note, in the table above, we have included daily limit for sucrose based on both the new suggestion from FDA of no more than 800 mg/kg a day of added sugars and the corresponding WHO diet recommendation (they call it ‘free sugars’) of 400-800 mg/kg per day (based on percent daily calories, calculated here for a woman weighing 60 kg). The European RI for total ‘sugars’ meanwhile is 1500 mg/kg, and the older US DRIs are a lot higher, limiting portion of added sugars to a maximum of about 2100 mg/kg day.
Limits of comparing toxicity metrics
Metrics are often misused to say “substance X is more or less toxic than substance Y”. However, even the best metrics are only a part of the story. Such oversimplified statements ignore whether we are talking about acute toxicity or chronic toxicity (people often reply to arguments about chronic toxicity with LD50 based comparisons). Because chemicals have different properties, sometimes a substance that is technically more acutely toxic can be less chronically toxic.
For example, in the charts in the piece, cyanide is more acutely toxic, with an LD50 of 4 mg/kg, than lead, with the lowest single lethal dose recorded at 155 mg/kg, almost 40 times higher. However, because lead accumulates in biological tissues, whereas cyanide does not, chronic low levels of lead add up and cause harm over time. This example highlights the difficulty in making blanket statements about the relative toxicity of different substances.
This is not to say that comparisons of toxicity metrics as indicators of general potential to cause harm are completely off base – often they can give some broad indications about general toxicity. MSG, sugar, glyphosate, and many other substances do keep their rough relative positions in both toxicity tables. Glyphosate remains one of the least harmful of pesticides in acute and chronic regards, while coffee is quite potently toxic in both. But these comparisons are only a rudimentary first sketch of the relative risk these substances pose to us.
Toxicity metrics also tell us nothing about actual exposures. In risk assessments, it is the comparison of metrics to likely or actual exposure levels that is important.
Update: an example of this kind of assessments here in a recent Danish paper on pesticide risk, which sets ADI levels in context with exposure data to arrive at a relative Hazard Index for four classes of substances which we have also touched on in our piece.
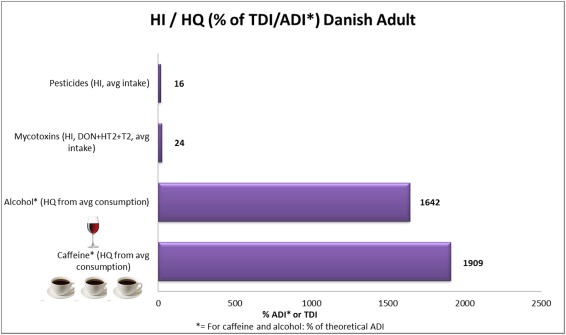
Risk assessment based on Accepted Daily Intake and exposure data from a Danish research article.
These metrics are benchmarks that scientists and regulators can use to guide risk assessment and mitigation. Metrics are also specific to their route of exposure. For example, oral and inhalation metrics are calculated differently – it is important to use the correct metric when making comparisons.
Safety limits are set very cautiously
Note that intakes above these limit levels are not necessarily very dangerous, especially not in the short term. Chronic toxicity metrics assume that daily consumption over a lifetime, so short-term exposure to a level higher than the reference dose can still be safe. For example, the reference dose for paracetamol (acetaminophen) is 0.093 mg/kg per day, which is 10 times below the actual therapeutic dose of 9.3 mg/kg. For short term use, this higher dose poses a minimal risk because it will not be taken daily over an entire lifetime.
For paracetamol the therapeutic level, which is intended for short term use, is equal to the LOAEL. In calculating the reference dose for chronic exposure purposes, regulatory agencies add a large safety margin to arrive at a daily limit of minimal concern (because the therapeutic dose, taken over long term, has been linked with an increase in liver enzyme levels).
That the safety limit of one substance is similar to another also does not mean that exceeding that limit for either of the substances would carry similar risks. Exceeding our daily Reference Dose for caffeine is commonplace, and the resulting adverse effects on heart rate, sleeping, and mood are small risks we are easily willing to take for the benefit of being alert, whereas exceeding the regulatory exposure limit to lead would offer no benefits, and exposure has very severe unwanted consequences.
Conclusions
These toxicity metrics are a critical starting point to help us begin to compare risks from different chemicals. We are continuously exposed to a great many substances, some of which may pose a threat if encountered in too high a concentration, too often, or via an inappropriate route of exposure (i.e. approved for dermal use but not oral ingestion). Meanwhile, many of these substances are necessary for us in their appropriate amounts, and while there are many others we don’t require, we easily tolerate them in low doses. It is important to remember that there are safe and unsafe levels of any substance, and that even regulatory ‘safe’ classifications always come with caveats of probability and context (like the type of use) as explored further by Alison in her article on How safe is safe?
Although no substance can be considered absolutely safe, a mere detection of a substance does not tell us whether its presence may pose a problem. For that, we must compare detections and exposure levels with these metrics to inform us about whether something actually poses a risk. The acute and chronic toxicity measurements highlighted in this piece both have their utility, but they can only set a benchmark for comparison to determine when a substance is or isn’t harmful.
For a better understanding of the likelihood of harm from exposures in between – including risks of trying to avoid exposure – we need to rely on proper risk assessments. Knowing how easy it is to intuitively jump into conclusions about risk, we have delved into the topic in depth in our series Risk In Perspective, and particularly: Zero Risk Is an Impossible Dream.

For more about some common concerns over substances in agriculture and medicine, you can find articles under Farming and GMOs and Vaccines and Health.
If you would like to have a discussion in the comments below, please take note of my Commenting policy. In a nutshell:
- Be respectful.
- Back up your claims with evidence.
References
For sources, please see the below tables for links. These raw tables include a couple more substances than included in the infographics (I cut a few from the infographics for size).
Unfortunately I realized that those links which lead to NIH Toxnet search results are temporary – but to find the references, you can just paste the substance name again into the search bar. Then use finder (ctrl+f) to look for the word “LD50” or “lethal” on the page.
Acute toxicity
Chronic toxicity
References to the chronic toxicity table values of RfD, ADI, RI, UL, TDI or TWI.


Excellent overview.
(However, your value for chronic toxicity of caffeine is incorrect—I personally consume about 1000-fold higher than the value you quote.)
LikeLike
Hello Peter Olins,
Thanks for stopping by, and for your kind words about the article.
You may check the RfD value for caffeine yourself from the sources provided, and a detailed explanation of how it was determined by Dr Bernstein, who has previously written about that specifically here: http://fafdl.org/blog/2017/04/13/glyphosate-vs-caffeine-acute-and-chronic-toxicity-assessments-explained/
Your exceeding the limit is not surprising. As we write here:
“That the safety limit of one substance is similar to another also does not mean that exceeding that limit for either of the substances would carry similar risks. Exceeding our daily Reference Dose for caffeine is commonplace, and the resulting adverse effects on heart rate, sleeping, and mood are small risks we are easily willing to take for the benefit of being alert”
I myself have landed at almost 1000 times the RfD dose today as well, and enjoyed the alertness of that.
Have a good day,
Iida/Thoughtscapism
LikeLike
Thanks. Brilliant article. Sadly, self-styled “environmentalists” are exactly those who insist on “Zero Risk” even while knowing that is impossible. It suits their political objectives to have the Public in a perpetual state of fear and anxiety.
It is very important that people who have REAL expertise and can relate to the public (like this Author) do everything they can, and never give up (!) to educate the public; appear on radio phone-ins, public debates, letters to editors and website comments, OF A meetings etc.
OR the Fear Mongers will win!!!
LikeLiked by 1 person
I think part of the reasons the public has had such difficulty with these concepts is the LACK of people like you and your friends to help translate science. I feel acutely the absence of qualified science writers and trained extension agents who are desperately needed to help people outside the labs understand how and why we make these decisions.
In the comment above, I don’t think scientists OR environmentalists appreciate being politicized. Scientists and environmentalists can both be wrong. I don’t know a single environmentalist whose agenda is about scaring people, but the level of unconcern and lack of human motivation without self-interest is the problem. Environmentalists do understand that it is far more efficient to keep evolved productive ecosystems intact than it is to try to put them back together once broken.
LikeLike
People who have a particular viewpoint and agenda, and want to persuade others, tend to be more interested in impressions than in facts. They like scary media articles, are bored by objective comparisons.
The news media is not our friend in this: They have to sell eyeballs to advertisers in order to survive, so they focus on whatever makes people excited.
People who don’t understand or care about the difference between anecdotes and peer review are their willing victims.
LikeLike
Agreed lovely piece. Worth submitting to one of the national newspapers (in each country?). The Guardian would be interested and hit a target audience
LikeLiked by 1 person
Reading these comments, It is easy, painfully easy, to see how low the functional literacy level is. Add to that the default of not just disbelief, but of paranoia that there is a plot hiding in every article. Translational science journalism has a hard road ahead!
LikeLike
an apology, I had this and a repost open. the comments I refer to are from the repost. these are the few that “get it” and a few on the repost, where the illiteracy is rampant. Sorry for the unintended insult!
LikeLike
Hello Walt!
No problem. I admit I was wondering a bit, I mean, I was rather happy about the comments here, but I wondered if you might have been referring to facebook – I whole-heartedly agreed with the sentiment concerning the posts I had made about this on my blog’s facebook page! I had quite a variety of comments. A lot of people were clearly not knowledgeable about toxicity metrics. Made me wonder about ways I could help make toxicity information more intuitive…
Thanks for reading and commenting!
Iida/Thoughtscapism
LikeLike
Pingback: The Dose Makes the Poison: 2016 edition | Camistry
Pingback: The dose makes the poison. | Camistry
Nice, well presented article. No discussion of toxicity is complete with out the words of the famous Swiss physician and alchemist, Paracelsus: “The dose makes the poison” (Latin: sola dosis facit venenum).
https://en.wikipedia.org/wiki/The_dose_makes_the_poison
Chemists consider him to be the first alchemist to begin the foundations of modern chemistry.
https://en.wikipedia.org/wiki/Paracelsus#Chemistry
LikeLike
Thank you for sharing this information.
LikeLiked by 1 person
Hello Thoughtscapism,
Excellent piece.
Regarding your comment: “Made me wonder about ways I could help make toxicity information more intuitive…”, I don’t think that’s possible, because science is not about intuition; it is precisely the opposite of intuition! So the only thing we can do is insist on science education, but when we are faced with people who will interrup us after half a sentence and blurt out “you known, I’m not interested in science”, it’s a bit disappointing..
Still I keep trying…
LikeLiked by 1 person
Hello Laurence,
I am very glad to hear you liked it!
I would argue that making information more intuitive is what any good lecturer or teacher does. Arriving at new understanding may not be intuitive, it may be wholly counter-intuitive, in fact, but more efficient learning about what science has uncovered can be done by presenting the chain of reasoning that leads one there in a way that makes it more intuitively relatable to the learner/listener.
As a simple example within the piece: relating the small risks from pesticide residues to risks from coffee and alcohol makes the information more intuitive, because we have a certain degree of intuition about the two latter substances (that is, we know that to get acute harm, we need large doses, and for long-term harm, at least we have the idea that we need considerable sustained intake, a sip now and then does practically nothing).
If people are not interested, there’s not much you can do, of course. If they are passionate about a topic, any topic, however, and insist on talking about it, then you can ask them if they do not think the topic important enough to warrant a really good, thorough look. That’s all that science really is. A really good thorough way of looking at something, if we are truly interested in finding out something about it.
Thanks for stopping by!
Iida/Thoughtscapism
LikeLiked by 1 person
Pingback: Toward More Intuitive Toxicology Information | Thoughtscapism
On the chronic dose table, I think there’s something hinky about the math for water and tylenol. 50,000 mg per kg is 50L per kg isn’t it?
And regular sized humans are dosed at 2500mg of tylenol per day – Maybe 40 mg/kg?
LikeLike
Hi Carl!
50 000 mg is 50 grams. That’s 50ml (about one fifth of a cup) per kg body weight. That’s 5 liters for a 100kg heavy person. Not strange that such a volume would start to cause adverse effects.
Hope this helps.
Iida/Thoughtscapism
LikeLike
Pingback: Chemical Exposures: The Good, the Bad, and the Tiny | Thoughtscapism